How Is Plant Cell Cytokinesis Different From Animal Cell Cytokinesis

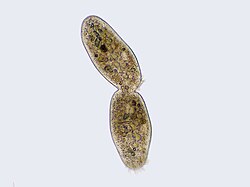
Cilliate undergoing cytokinesis, with the cleavage furrow being clearly visible.
Cytokinesis () is the function of the jail cell sectionalisation process during which the cytoplasm of a unmarried eukaryotic cell divides into two daughter cells. Cytoplasmic division begins during or later on the late stages of nuclear division in mitosis and meiosis. During cytokinesis the spindle appliance partitions and transports duplicated chromatids into the cytoplasm of the separating daughter cells. It thereby ensures that chromosome number and complement are maintained from one generation to the next and that, except in special cases, the girl cells will be functional copies of the parent jail cell. After the completion of the telophase and cytokinesis, each daughter cell enters the interphase of the cell bike.
Particular functions demand various deviations from the process of symmetrical cytokinesis; for example in oogenesis in animals the ovum takes almost all the cytoplasm and organelles. This leaves very niggling for the resulting polar bodies, which in most species die without function, though they practise have on diverse special functions in other species.[1] Another class of mitosis occurs in tissues such as liver and skeletal musculus; information technology omits cytokinesis, thereby yielding multinucleate cells.
Found cytokinesis differs from creature cytokinesis, partly because of the rigidity of constitute jail cell walls. Instead of plant cells forming a cleavage furrow such as develops betwixt animate being girl cells, a dividing construction known as the cell plate forms in the cytoplasm and grows into a new, doubled prison cell wall betwixt plant daughter cells. It divides the cell into two daughter cells.
Cytokinesis largely resembles the prokaryotic procedure of binary fission, simply because of differences between prokaryotic and eukaryotic cell structures and functions, the mechanisms differ. For case, a bacterial cell has a Circular chromosome (a single chromosome in the course of a closed loop), in dissimilarity to the linear, ordinarily multiple, chromosomes of eukaryote. Accordingly, bacteria construct no mitotic spindle in jail cell partitioning. Also, duplication of prokaryotic DNA takes place during the actual separation of chromosomes; in mitosis, duplication takes identify during the interphase earlier mitosis begins, though the daughter chromatids exercise not separate completely earlier the anaphase.
Etymology and pronunciation [edit]
The word "cytokinesis" ([2] [3]) uses combining forms of cyto- + kine- + -sis, New Latin from Classical Latin and Ancient Greek, reflecting "cell" and kinesis ("motility, motion"). It was coined by Charles Otis Whitman in 1887.[iv]
Origin of this term is from Greek κύτος ( kytos , a hollow), Latin derivative cyto (cellular), Greek κίνησις ( kínesis , movement).
Animal jail cell [edit]
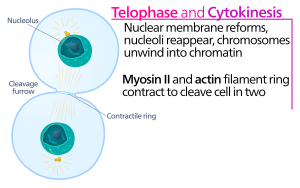
Animal prison cell cytokinesis begins shortly subsequently the onset of sister chromatid separation in the anaphase of mitosis. The process tin be divided to the following singled-out steps: anaphase spindle reorganization, partitioning plane specification, actin-myosin ring assembly and contraction, and abscission.[5] Faithful partitioning of the genome to emerging girl cells is ensured through the tight temporal coordination of the above individual events by molecular signaling pathways.
Anaphase spindle reorganization [edit]
Animal cell cytokinesis starts with the stabilization of microtubules and reorganization of the mitotic spindle to grade the central spindle. The central spindle (or spindle midzone) forms when non-kinetochore microtubule fibers are arranged between the spindle poles. A number of unlike species including H. sapiens, D. melanogaster and C. elegans require the central spindle in order to efficiently undergo cytokinesis, although the specific phenotype associated with its absence varies from one species to the next (for example, certain Drosophila cell types are incapable of forming a cleavage furrow without the central spindle, whereas in both C. elegans embryos and human tissue culture cells a cleavage furrow is observed to grade and ingress, just then regress before cytokinesis is complete). The process of mitotic spindle reorganization and central spindle formation is caused by the pass up of CDK1 action during anaphase.[5] The decline of CDK1 activity at the metaphase-anaphase transition leads to dephosphorylating of inhibitory sites on multiple cardinal spindle components. First of all, the removal of a CDK1 phosphorylation from a subunit of the CPC (the chromosomal passenger complex) allows its translocalization to the cardinal spindle from the centromeres, where it is located during metaphase. Too beingness a structural component of the cardinal spindle itself, CPC also plays a role in the phosphoregulation of other central spindle components, including PRC1 (microtubule-bundling poly peptide required for cytokinesis 1) and MKLP1 (a kinesin motor protein). Originally inhibited past CDK1-mediated phosphorylation, PRC1 is now able to form a homodimer that selectively binds to the interface betwixt antiparallel microtubules, facilitating spatial organization of the microtubules of the central spindle. MKLP1, together with the Rho-family GTPase activating poly peptide CYK-iv (also termed MgcRacGAP), forms the centralspindlin complex. Centralspindlin binds to the central spindle every bit college-order clusters. The centralspindlin cluster formation is promoted by phosphorylation of MLKP1 by Aurora B, a component of CPC. In brusque, the self-assembly of central spindle is initiated through the phosphoregulation of multiple central spindle components by the decline of CDK1 action, either directly or indirectly, at the metaphase-anaphase transition. The central spindle may have multiple functions in cytokinesis including the control of cleavage furrow positioning, the commitment of membrane vesicles to the cleavage furrow, and the formation of the midbody structure that is required for the terminal steps of sectionalization.[6]
Division plane specification [edit]
The second stride of animal cell cytokinesis involves partitioning plane specification and cytokinetic furrow formation. Precise positioning of the division plane between the 2 masses of segregated chromosomes is essential to foreclose chromosome loss. Meanwhile, the mechanism past which the spindle determines the division plane in beast cells is perchance the most enduring mystery in cytokinesis and a matter of intense contend. There be three hypotheses of furrow consecration.[half-dozen] The first is the astral stimulation hypothesis, which postulates that astral microtubules from the spindle poles conduct a furrow-inducing point to the jail cell cortex, where signals from two poles are somehow focused into a band at the spindle. A second possibility, called the key spindle hypothesis, is that the cleavage furrow is induced by a positive stimulus that originates in the central spindle equator. The central spindle may contribute to the specification of the partitioning airplane by promoting concentration and activation of the small GTPase RhoA at the equatorial cortex. A third hypothesis is the astral relaxation hypothesis. It postulates that active actin-myosin bundles are distributed throughout the prison cell cortex, and inhibition of their contraction almost the spindle poles results in a gradient of contractile action that is highest at the midpoint betwixt poles. In other words, astral microtubules generate a negative signal that increases cortical relaxation close to the poles. Genetic and laser-micromanipulation studies in C. elegans embryos accept shown that the spindle sends two redundant signals to the cell cortex, one originating from the cardinal spindle, and a second signal deriving from the spindle aster, suggesting the involvement of multiple mechanisms combined in the positioning of the cleavage furrow. The predominance of i particular betoken varies betwixt cell types and organisms. And the multitude and partial redundancy of signals may be required to make the organization robust and to increase spatial precision.[5]
Actin-myosin ring associates and contraction [edit]
At the cytokinesis furrow, it is the actin-myosin contractile band that drives the cleavage process, during which cell membrane and wall abound inward, which somewhen pinches the mother cell in two. The key components of this ring are the filamentous protein actin and the motor protein myosin 2. The contractile band assembles equatorially (in the middle of the cell) at the cell cortex (adjacent to the jail cell membrane). Rho protein family (RhoA protein in mammalian cells) is a key regulator of contractile ring germination and contraction in animal cells.[6] The RhoA pathway promotes assembly of the actin-myosin ring by two main effectors. First, RhoA stimulates nucleation of unbranched actin filaments past activation of Diaphanous-related formins. This local generation of new actin filaments is important for the contractile ring germination.[6] This actin filament formation process likewise requires a protein called profilin, which binds to actin monomers and helps load them onto the filament finish. 2nd, RhoA promotes myosin 2 activation by the kinase Rock, which activates myosin II direct by phosphorylation of the myosin light chain and also inhibits myosin phosphatase by phosphorylation of the phosphatase-targeting subunit MYPT. Besides actin and myosin II, the contractile ring contains the scaffolding protein anillin. Anillin binds to actin, myosin, RhoA, and CYK-4, and thereby links the equatorial cortex with the signals from the central spindle. It too contributes to the linkage of the actin-myosin band to the plasma membrane. Additionally, anillin generates contractile forces by rectifying thermal fluctuations.[7] Another protein, septin, has too been speculated to serve as a structural scaffold on which the cytokinesis appliance is organized. Following its assembly, wrinkle of the actin-myosin band leads to ingression of the attached plasma membrane, which partitions the cytoplasm into two domains of emerging sis cells. The force for the contractile processes is generated past movements along actin by the motor protein myosin Ii. Myosin II uses the free energy released when ATP is hydrolyzed to move along these actin filaments, constricting the cell membrane to form a cleavage furrow. Continued hydrolysis causes this cleavage furrow to ingress (move inwards), a hit process that is conspicuously visible through a light microscope.
Abscission [edit]
The cytokinetic furrow ingresses until a midbody construction (composed of electron-dense, proteinaceous material) is formed, where the actin-myosin band has reached a diameter of about 1–ii μm. Nearly animal cell types remain connected by an intercellular cytokinetic span for up to several hours until they are split by an actin-independent process termed abscission, the last step of cytokinesis.[five] [8]
The process of abscission physically cleaves the midbody into two. Abscission proceeds by removal of cytoskeletal structures from the cytokinetic bridge, constriction of the cell cortex, and plasma membrane fission. The intercellular bridge is filled with dense bundles of antiparallel microtubules that derive from the cardinal spindle. These microtubules overlap at the midbody, which is generally thought to be a targeting platform for the abscission machinery.
The microtubule severing poly peptide spastin is largely responsible for the disassembly of microtubule bundles within the intercellular bridge. Complete cortical constriction likewise requires removal of the underlying cytoskeletal structures. Actin filament disassembly during belatedly cytokinesis depends on the PKCε–fourteen-3-3 complex, which inactivates RhoA after furrow ingression. Actin disassembly is further controlled past the GTPase Rab35 and its effector, the phosphatidylinositol-iv,5-bisphosphate five-phosphatase OCRL. Agreement the mechanism by which the plasma membrane ultimately splits requires further investigation.
Timing cytokinesis [edit]
Cytokinesis must be temporally controlled to ensure that it occurs but after sister chromatids separate during the anaphase portion of normal proliferative cell divisions. To achieve this, many components of the cytokinesis mechanism are highly regulated to ensure that they are able to perform a item office at merely a item phase of the jail cell wheel.[ix] [10] Cytokinesis happens only later APC binds with CDC20. This allows for the separation of chromosomes and myosin to work simultaneously.
Later on cytokinesis, not-kinetochore microtubules reorganize and disappear into a new cytoskeleton as the cell bike returns to interphase (run into also prison cell cycle).
Found cell [edit]
Due to the presence of a jail cell wall, cytokinesis in constitute cells is significantly different from that in creature cells, Rather than forming a contractile band, found cells construct a prison cell plate in the eye of the cell. The stages of cell plate formation include (i) cosmos of the phragmoplast, an array of microtubules that guides and supports the formation of the cell plate; (2) trafficking of vesicles to the sectionalization aeroplane and their fusion to generate a tubular-vesicular network; (3) continued fusion of membrane tubules and their transformation into membrane sheets upon the deposition of callose, followed by deposition of cellulose and other jail cell wall components; (4) recycling of excess membrane and other material from the cell plate; and (five) fusion with the parental cell wall[11] [12]
The phragmoplast is assembled from the remnants of the mitotic spindle, and serves as a track for the trafficking of vesicles to the phragmoplast midzone. These vesicles incorporate lipids, proteins and carbohydrates needed for the germination of a new cell boundary. Electron tomographic studies accept identified the Golgi appliance as the source of these vesicles,[thirteen] [14] just other studies have suggested that they contain endocytosed textile equally well.[15] [16]
These tubules and then widen and fuse laterally with each other, eventually forming a planar, fenestrated sail [8]. As the jail cell plate matures, large amounts of membrane material are removed via clathrin-mediated endocytosis [7] Somewhen, the edges of the cell plate fuse with the parental plasma membrane, ofttimes in an asymmetrical mode,[17] thus completing cytokinesis. The remaining fenestrae contain strands of endoplasmic reticulum passing through them, and are thought to be the precursors of plasmodesmata [8].
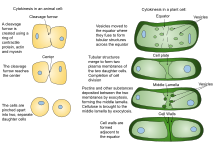
The process of Cytokinesis in a plant cell and an creature cell
The construction of the new cell wall begins within the lumen of the narrow tubules of the young cell plate. The order in which unlike cell wall components are deposited has been determined largely by immuno-electron microscopy. The offset components to arrive are pectins, hemicelluloses, and arabinogalactan proteins carried by the secretory vesicles that fuse to form the prison cell plate.[18] The next component to exist added is callose, which is polymerized direct at the cell plate by callose synthases. As the prison cell plate continues to mature and fuses with the parental plasma membrane, the callose is slowly replaced with cellulose, the primary component of a mature cell wall [6]. The middle lamella (a glue-like layer containing pectin) develops from the prison cell plate, serving to demark the cell walls of bordering cells together.[19] [xx]
Forces [edit]
Creature cells [edit]
Cytokinetic furrow ingression is powered by Type II Myosin ATPase. Since Myosins are recruited to the medial region, the contractile forces acting on the cortex resemble a 'pocketbook string' constriction pulling in. This leads to the inward constriction. The plasma membrane by virtue of its shut association with the cortex via crosslinker proteins [21] To the constriction of the cleavage furrow, the total surface expanse should be increased past supplying the plasma membrane via exocytosis </ref>.[22]
Proteins involved in cytokinesis [edit]
CEP55 is a mitotic phosphoprotein that plays a key role in cytokinesis, the concluding phase of cell divisionthe final stage of cell division.[23] [24]
See as well [edit]
- Diploid
- Telophase – Final stage of a prison cell sectionalisation for eukaryotic cells both in mitosis and meiosis
- Prophase – First phase of cell partitioning in both mitosis and meiosis
- Anaphase – Stage of a cell segmentation
- Metaphase – Stage of cell division
- Mitosis – Process in which replicated chromosomes are separated into 2 new identical nuclei
- Jail cell theory – Biology of cells
- Cytoskeleton – Network of filamentous proteins that forms the internal framework of cells
References [edit]
- ^ Schmerler Samuel, Wessel Gary (January 2011). "Polar Bodies - more a lack of understanding than a lack of respect". Mol Reprod Dev. 78 (1): 3–8. doi:10.1002/mrd.21266. PMC3164815. PMID 21268179.
- ^ "cytokinesis". Oxford Dictionaries UK English Dictionary. Oxford University Printing. northward.d. Retrieved 2016-01-21 .
- ^ "cytokinesis". Merriam-Webster Dictionary . Retrieved 2016-01-21 .
- ^ Battaglia, Emilio (2009). Caryoneme alternative to chromosome and a new caryological nomenclature. Caryologia 62 (4): 1–80. link.
- ^ a b c d Fededa JP, Gerlich DW (May 2012). "Molecular command of beast jail cell cytokinesis". Nat. Prison cell Biol. fourteen (5): 440–7. doi:10.1038/ncb2482. PMID 22552143. S2CID 3355851.
- ^ a b c d Morgan, David (2007). The Prison cell Cycle. New Science Press. pp. 157–173.
- ^ Kucera, Ondrej; Siahaan, Valerie; Janda, Daniel; Dijkstra, Sietske H; Pilatova, Eliska; Zatecka, Eva; Diez, Stefan; Braun, Marcus; Lansky, Zdenek (2021). "Anillin propels myosin-contained constriction of actin rings". Nature Communications. 12 (i): 4595. Bibcode:2021NatCo..12.4595K. doi:10.1038/s41467-021-24474-1. PMC8319318. PMID 34321459.
- ^ "Cytokinetic bridge". proteinatlas.org . Retrieved 28 August 2019.
- ^ Mishima 1000, Pavicic 5, Grüneberg U, Nigg EA, Glotzer M (August 2004). "Cell cycle regulation of central spindle assembly". Nature. 430 (7002): 908–13. Bibcode:2004Natur.430..908M. doi:10.1038/nature02767. PMID 15282614. S2CID 4418281.
- ^ Petronczki M, Glotzer Grand, Kraut N, Peters JM (May 2007). "Polo-similar kinase 1 triggers the initiation of cytokinesis in human cells past promoting recruitment of the RhoGEF Ect2 to the central spindle". Dev. Cell. 12 (5): 713–25. doi:10.1016/j.devcel.2007.03.013. PMID 17488623.
- ^ Otegui M, Staehelin LA (December 2000). "Cytokinesis in flowering plants: more than one way to split up a cell". Curr. Opin. Institute Biol. 3 (6): 493–502. doi:10.1016/s1369-5266(00)00119-9. PMID 11074381.
- ^ Samuels AL, Giddings Th, Staehelin LA (September 1995). "Cytokinesis in tobacco BY-2 and root tip cells: a new model of jail cell plate germination in higher plants". J. Cell Biol. 130 (6): 1345–57. doi:ten.1083/jcb.130.6.1345. PMC2120572. PMID 7559757.
- ^ Otegui MS, Mastronarde DN, Kang BH, Bednarek SY, Staehelin LA (September 2001). "3-dimensional assay of syncytial-blazon cell plates during endosperm cellularization visualized by loftier resolution electron tomography". Plant Prison cell. thirteen (9): 2033–51. doi:x.1105/tpc.thirteen.9.2033. PMC139450. PMID 11549762.
- ^ Seguí-Simarro JM, Austin JR, White EA, Staehelin LA (April 2004). "Electron tomographic analysis of somatic cell plate germination in meristematic cells of Arabidopsis preserved by loftier-pressure freezing". Plant Jail cell. xvi (4): 836–56. doi:ten.1105/tpc.017749. PMC412860. PMID 15020749.
- ^ Baluska F, Liners F, Hlavacka A, Schlicht One thousand, Van Cutsem P, McCurdy DW, Menzel D (October 2005). "Jail cell wall pectins and xyloglucans are internalized into dividing root cells and accumulate inside jail cell plates during cytokinesis". Protoplasma. 225 (iii–4): 141–55. doi:10.1007/s00709-005-0095-v. PMID 16228896. S2CID 11881080.
- ^ Dhonukshe P, Baluska F, Schlicht M, Hlavacka A, Samaj J, Friml J, Gadella TW (January 2006). "Endocytosis of cell surface material mediates cell plate formation during plant cytokinesis". Dev. Cell. 10 (1): 137–l. doi:ten.1016/j.devcel.2005.eleven.015. PMID 16399085.
- ^ Cutler SR, Ehrhardt DW (March 2002). "Polarized cytokinesis in vacuolate cells of Arabidopsis". Proc. Natl. Acad. Sci. U.Due south.A. 99 (5): 2812–7. Bibcode:2002PNAS...99.2812C. doi:10.1073/pnas.052712299. PMC122430. PMID 11880633.
- ^ Staehelin LA, Moore I (1995). "The Plant Golgi Appliance: Structure, Functional Organization and Trafficking Mechanisms". Annual Review of Constitute Physiology and Institute Molecular Biology. 46 (1): 261–288. doi:10.1146/annurev.pp.46.060195.001401. ISSN 1040-2519.
- ^ Charles E. Allen (July 1901). "On the Origin and Nature of the Eye Lamella". Botanical Gazette. 32 (ane): 1–34. doi:10.1086/328131. JSTOR 2464904. S2CID 84936099.
- ^ Evert RF, Eichorn Due south (2006-09-xviii). Esau's Plant Beefcake: Meristems, Cells, and Tissues of the Plant Body: Their Structure, Part, and Development. John Wiley & Sons. ISBN978-0-470-04737-8.
- ^ Alberts B, Johnson A, Lewis J, Raff Grand, Roberts M, Walter P (2008-06-18). "Cross-linking Proteins with Distinct Properties Organize Different Assemblies of Actin Filaments" - Molecular Biology of the Prison cell, 4th Ed, 2002: Cell. Garland Science. pp. 1006–. ISBN978-0-8153-3218-3.
- ^ Tanaka G, Fujimoto K, Yumura Due south (April 2020). "Regulation of the Total Cell Surface Area in Dividing Dictyostelium Cells". Front Cell Dev Biol. 8: 238. doi:10.3389/fcell.2020.00238. PMC7156592. PMID 32322581.
- ^ van der Horst A, Simmons J, Khanna KK (November 2009). "Cep55 stabilization is required for normal execution of cytokinesis". Cell Cycle. 8 (22): 3742–ix. doi:x.4161/cc.eight.22.10047. PMID 19855176.
- ^ Behnam Rashidieh,Belal Shohayeb,Amanda Louise Bain,Patrick R. J. Fortuna,Debottam Sinha,Andrew Burgess,Richard Mills,Rachael C. Adams,J. Alejandro Lopez,Peter Blumbergs,John Finnie,Murugan Kalimutho,Michael Piper,James Edward Hudson,Dominic C. H. Ng ,Kum Kum Khanna. (Oct 2021). "Cep55 regulation of PI3K/Akt signaling is required for neocortical development and ciliogenesis". PLoS Genetics. doi:10.1371/periodical.pgen.1009334. PMID 34710087.
{{cite journal}}: CS1 maint: uses authors parameter (link)
Farther reading [edit]
- The Molecular Requirements for Cytokinesis past 1000. Glotzer (2005), Scientific discipline 307, 1735
- "Animal Cytokinesis: from parts list to mechanism" by Eggert, U.Due south., Mitchison, T.J., Field, C.M. (2006), Annual Review of Cell Biology 75, 543-66
- Campbell Biology (2010), 580-582
- More description and nice images of cell partition in plants, with a focus on fluorescence microscopy
- Nanninga N (June 2001). "Cytokinesis in Prokaryotes and Eukaryotes: Mutual Principles and Different Solutions". Microbiol. Mol. Biol. Rev. 65 (2): 319–33. doi:10.1128/MMBR.65.ii.319-333.2001. PMC99029. PMID 11381104.
Source: https://en.wikipedia.org/wiki/Cytokinesis
Posted by: frostdescear.blogspot.com

0 Response to "How Is Plant Cell Cytokinesis Different From Animal Cell Cytokinesis"
Post a Comment